OTC Drug Labels Attorney in Miami, Florida
FDA regulates the required labeling on an OTC drug product through regulations referred to as monographs. The monographs list the labeling requirements, active ingredients, and testing requirements per the OTC drug product category.
Ensure Your Drug Labels are Compliant with FDA Regulations
REACH OUT NOW
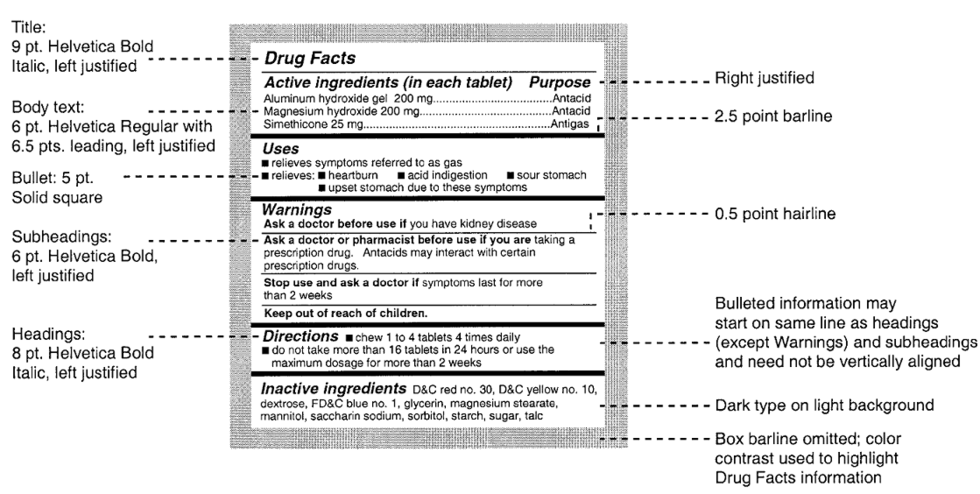
FDA regulations specify the font size, thickness of lines, and whether typeset must be bold or regular font. Below is a sample Drug Facts panel showing the font sizes and the formatting that is required. This is a starting point for any graphic artist that is designing an OTC label.